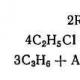Sections of the site
Editor's Choice:
- The size of the money supply. Money supply. money supply indicators. The structure of the money supply
- Scientific electronic library
- Certification of workplaces has been replaced by a special assessment of working conditions Examination of workplaces
- Abstract: Environmental pollution
- Rules for archival storage of documents
- Bags under the eyes - causes and methods of elimination If a water bag has formed under the eye
- Fairy tale shark for children - lion tolstoy
- L. Tolstoy. Shark outline of a lesson in reading (Grade 3) on the topic. Fairy tale shark for children - Leo Tolstoy Who wrote the shark the author of the fairy tale
- Conversation about healthy eating Conversations with students about the benefits of healthy eating
- Vasily Filippovich Margelov
Advertising
| Benzene poisoning symptoms. Alkylation at a carbon atom |
|
Alkylation is the process of introducing alkyl groups into the molecules of organic and some inorganic substances. These reactions are of great practical importance for the synthesis of aromatic compounds alkylated into the nucleus, isoparaffins, many mercaptans and sulfides, amines, substances with ether bonds, elemental and organometallic compounds, products of processing of α-oxides and acetylene. Alkylation processes are often intermediate steps in the production of monomers, detergents, etc. CHARACTERISTICS OF ALKYLATION PROCESSES Classification of alkylation reactions. The most rational classification of alkylation processes is based on the type of newly formed bond. Alkylmoatanieonatom carbon(C-alkyliroing) consists in replacing the hydrogen atom located at the carbon atom with an alkyl group. Paraffins are capable of this substitution, but alkylation is most characteristic for aromatic compounds (the Friedel-Crafts reaction): Atomic Alkylationoxygenandsulfur(Oh- andS-alkylation) is a reaction in which an alkyl group binds to an oxygen or sulfur atom: Alkylation byatom nitrogen (N-alkylation) consists in the replacement of hydrogen atoms in ammonia or in amines by alkyl groups. This is the most important of the methods for the synthesis of amines: As in the case of hydrolysis and hydration reactions, N-alkylation is often classified as ammonolysis (or aminolysis) of organic compounds. Alkylation onatoms others elements(Si-, Pb-, A1-alkylation) is the most important way to obtain elemental and organometallic compounds, when the alkyl group is directly bonded to the hetero-atom:
Another classification of alkylation reactions is based on differences in the structure of the alkyl group introduced into an organic or inorganic compound. The alkyl group may be saturated aliphatic (eg ethyl and isopropyl) or cyclic. In the latter case, the reaction is sometimes called cycloalkylation:
With the introduction of a phenyl or aryl group in general, a direct bond is formed with the carbon atom of the aromatic nucleus - arylation: Introduction of the vinyl group (vinylation) occupies a special place and is carried out mainly with the help of acetylene:
The most important of the reactions of introducing substituted alkyl groups is the process β-oxyalkeelandmoatania(in a particular case oxystylation), covering a wide range of reactions of olefin oxides: Alkylating agents and catalysts. All alkylating agents according to the type of bond that breaks in them during alkylation, it is advisable to divide into the following groups: unsaturated compounds (olefins and acetylene), which break the π-electronic bond between carbon atoms; chlorine derivatives with a sufficiently mobile chlorine atom capable of being replaced under the influence of various agents; alcohols, ethers and esters, in particular olefin oxides, in which the carbon-oxygen bond is broken during alkylation. Olefins(ethylene, propylene, butenes and higher propylene trimmers) are of paramount importance as alkylating agents. Due to their cheapness, they try to use them in all cases where possible. They have found their main application for the C-alkylation of paraffins and aromatic compounds. They are not applicable for N-alkylation and are not always effective in S- and O-alkylation and in the synthesis of organometallic compounds. Alkylation with olefins in most cases proceeds according to the ionic mechanism through the intermediate formation of carbocations and is catalyzed by protic and aprotic acids: The reactivity of olefins in reactions of this type is determined by their tendency to form carbocations: This means that the elongation and branching of the carbon chain in the olefin significantly increases its ability to alkylate. Chlorine derivatives are alkylating agents of the widest range of action. They are suitable for C-, O-, S- and N-alkylation and for the synthesis of most elemental and organometallic compounds. The use of chlorine derivatives is rational for those processes in which they cannot be replaced by olefins or when chlorine derivatives are cheaper and more accessible than olefins. The alkylating action of chlorine derivatives manifests itself in three different types of interactions: in electrophilic reactions, in nucleophilic substitution, and in free radical processes. The mechanism of electrophilic substitution is characteristic mainly for alkylation at the carbon atom, but, unlike olefins, reactions are catalyzed only by aprotic acids (aluminum and iron chlorides). In the limiting case, the process proceeds with the intermediate formation of a carbocation in this connection, the reactivity of alkyl chlorides depends on the polarization of the C-C1 bond or on the stability of carbocations and increases with elongation and branching of the alkyl group: CH3-CH 2 C1< (СН 3) 2 СНС1 < (СН 3) 3 СС1 Alcohols and ethers capable of C-, O-, N- and S-alkylation reactions. Olefin oxides, which are internal esters of glycols, can also be classified as ethers, and of all ethers, only olefin oxides are practically used as alkylating agents. Alcohols are used for O- and N-alkylation when they are cheaper and more accessible than chlorine derivatives. To break their alkyl-oxygen bond, acid-type catalysts are required: ALKYLATION AT THE CARBON ATOM The reactions of alkylation of aromatic compounds into the nucleus and the alkylation of paraffins, which are very important in practical terms, belong to processes of this type. More generally, they can be divided into aromatic and saturated carbon alkylation processes. reaction mechanism. As alkylating agents in industry, mainly chlorine derivatives and ^ole^ins are used. The use of alcohols is less efficient, because during alkylation with alcohols, aluminum chloride decomposes, and protic acids are diluted with the resulting water. In both cases, the catalyst is deactivated, which causes its high consumption. When reacting with chlorine derivatives or olefins, A1C1 3 is consumed only in catalytic amounts. In the first case, it activates the chlorine atom, forming a highly polarized complex or carbocation, which occurs with olefins only in the presence of a cocatalyst - HC1: In fact, during catalysis by a complex of aluminum chloride with a hydrocarbon, the proton required for this is already present in the form of an a-complex. It is transferred to the olefin molecule, and the resulting carbocation attacks the aromatic compound, and the entire reaction takes place in the layer of the catalytic complex, which continuously exchanges its ligands with the hydrocarbon layer. The carbocation obtained in one way or another (or a strongly polarized complex) then attacks the aromatic nucleus, and the reaction proceeds through the intermediate n-complex and carbocation, followed by the rapid stage of proton elimination: The structure of the alkyl group in the resulting product is determined by the rule of intermediate formation of the most stable carbocation (tert-> second-> re-). Therefore, in the case of lower olefins, primary alkylbenzene (ethylbenzene) is formed only from ethylene, secondary (isopropylbenzene) from propylene, and tert-butylbenzene from isobutene:
However, during alkylation with higher olefins and chlorine derivatives, isomerization of alkyl groups is observed, which occurs before alkylation, since alkylbenzenes are no longer capable of it. This isomerization proceeds in the direction of the intermediate formation of the most stable carbocation, but without breaking the carbon skeleton of the alkyl group, but only with the displacement of the reaction center. As a result, a mixture of sec-alkylbenzenes is obtained from chlorine derivatives and olefins with a straight chain of carbon atoms
and from compounds with a branched chain - mainly tert- alkylbenzenes. The influence of the structure of an aromatic compound in alkylation reactions is generally the same as in other processes of electrophilic substitution into an aromatic nucleus, but has its own characteristics. The alkylation reaction is characterized by relatively low sensitivity to electron-donating substituents in the nucleus. Thus, the activating effect of alkyl groups and condensed nuclei during the catalysis of the A1C1 3 reaction changes as follows (for benzene, the value is taken as 1): Electron-withdrawing substituents strongly deactivate the aromatic nucleus. Chlorobenzene is alkylated about 10 times slower than benzene, and carbonyl, carboxy, cyano, and nitro groups lead to complete deactivation of the aromatic nucleus, as a result of which the corresponding derivatives are not capable of alkylation at all. This alkylation reaction differs significantly from other substitution processes into an aromatic nucleus, such as chlorination and sulfonation. Alkylation orientation rules are generally similar to other electrophilic aromatic substitution reactions, but the structure of the product can vary significantly depending on catalysts and reaction conditions. Thus, electron-donating substituents and halogen atoms direct further substitution mainly to pair- and ortho- position, however, under more stringent conditions, and especially during catalysis with aluminum chloride, isomerization of benzene homologues occurs with intramolecular migration of alkyl groups and the formation of equilibrium mixtures in which thermodynamically more stable isomers predominate. sequential alkylation. When aromatic compounds are alkylated in the presence of any catalysts, successive substitution of hydrogen atoms occurs with the formation of a mixture of products of different degrees of alkylation. For example, methylation and ethylation of benzene goes up to the production of hexaalkylbenzenes propylation - to obtain tetraisopropylbenzene, etc. Each of the reactions at a moderate temperature is practically irreversible. Thus, the equilibrium constants in the synthesis of ethylbenzene from ethylene and benzene at 0, 200 and 500 °C are 6-10 11 , 2.2-10 4 and 1.9, respectively. However, during AlCl3 catalysis and sufficiently severe conditions of catalysis with aluminosilicates and zeolites, a reversible transalkylation reaction (disproportionation) occurs with intermolecular migration of alkyl groups:With the same catalysts, the reversible isomerization discussed above proceeds with intramolecular migration of alkyl groups, as a result of which the meta-isomer predominates among dialkylbenzenes, the 1,3,5-isomer among trialkylbenzenes, etc.:
The ability of alkyl groups to migrate changes in the following sequence (CH 3) 3 C > (CH 3) 2 CH > CH 3 -CH 2 > CH 3, and with the active complex of aluminum chloride, these reactions proceed quite quickly already at room temperature, while while methylbenzenes require prolonged heating. Thus, during catalysis with protic acids, and under milder conditions with other catalysts, the composition of alkylation products is determined by kinetic factors, while with AlCl 3 and under more severe conditions of catalysis with aluminosilicates and zeolites, an equilibrium composition of isomers and products of sequential alkylation can be established in the limit. This is of great importance when choosing the optimal molar ratio of reagents in alkylation, which is determined by the economic costs of the formation of polyalkylbenzenes and the return of excess benzene. Adverse reactions. In addition to the education discussed earlier polyalkylbenzenes during alkylation, resin formation, destruction of alkyl groups and polymerization of olefins are undesirable. Resin formation consists in obtaining condensed aromatic compounds with a high boiling point. Diarylalkanes, triarylindanes, diarylindanes, diarylolefins, etc., were found from similar products during the alkylation of benzene. During the alkylation of naphthalene, more resin is obtained, and dinaphthyl and other substances with condensed cycles are found in it. Resin formation becomes especially significant with increasing temperature. These same conditions lead to undesirable degradation of alkyl groups and the by-product formation of alkylbenzenes with a shorter alkyl group. So, when reacting with propylene, ethylbenzene is obtained as a by-product, with ethylene - toluene, etc. Such destruction is especially noticeable during alkylation with alkyl halides and olefins with a sufficiently long carbon chain. Destruction probably occurs at the stage of cleavage of the carbocation formed from the alkylating agentFinally, the formation of polymers occurs as a result of the sequential interaction of the carbocation with the olefin: The polymers have a low molecular weight and their formation is suppressed by the presence of an excess of aromatic hydrocarbon with a decrease in the concentration of olefin in the liquid phase. Kinetics of the process. The alkylation reaction itself with an active aluminum chloride complex proceeds very rapidly, is greatly accelerated by mechanical stirring or intensive bubbling of gaseous olefins through the reaction mass, and proceeds in the diffusion region or close to it. Its rate increases with increasing pressure, but depends little on temperature, having a low activation energy. At the same time, the usual dependence in the reactivity of olefins is preserved - stronger than the difference in their solubility. Apparently, the stage of olefin diffusion through the boundary film of the aluminum chloride catalytic complex, in which all reactions proceed, is the limiting one. In contrast, transalkylation proceeds much more slowly and accelerates significantly with increasing temperature, since it has an activation energy of ~63kJ/mol. Both reactions are slowed down by the gradual deactivation of the catalyst, but the rate of transalkylation drops especially strongly. As a result, a significant amount of polyalkylbenzenes will accumulate in the reaction mixture, which do not have time to enter into a reversible transalkylation reaction. To avoid this, it is necessary to limit the supply of reagents, and, consequently, the possibility of intensifying the process is limited by the slowest transalkylation reaction. In addition to reagent impurities, the deactivation of the catalyst is affected by the accumulation of certain alkylation by-products that are capable of firmly binding AlCl 3 or forming stable σ-complexes that donate their proton to the olefin molecule with difficulty. Such substances at low temperatures, when transalkylation proceeds slowly, are polyalkylbenzenes, and at high temperatures, polycyclic aromatic compounds and resins. As a result, it turns out that the optimal performance and consumption of the catalyst in the production of ethyl and isopropylbenzene are achieved at a certain average temperature (“100°C”), when the transalkylation proceeds quite quickly, but there are still few polycyclic substances that deactivate the catalyst. In the synthesis of compounds with a longer alkyl group, the choice of temperature is limited by the side reaction of degradation, and in the production of alkylnaphthalenes, by the processes of condensation and resinification. In these cases, its optimum is 30-50 °C, and in the case of alkylation of naphthalene, the selectivity can be further increased by using a solvent. This is due to the fact that in the reaction system
Resin formation is of the second order in terms of naphthalene or yal-kilnaphthalene, and the main reaction is the first. As a result, the selectivity for alkylnaphthalene increases with decreasing naphthalene concentration. Technological basis of the process Since the transalkylation reaction proceeds in the alkylator simultaneously with alkylation, for the joint carrying out of these processes, the DEBs (PABs) fraction isolated from the reaction mass during rectification is also fed into the alkylator together with benzene and ethylene. Since this process takes place in the diffusion region, it is necessary to use a bubbler to increase the interface; The reaction proceeds with the release of heat, therefore it is necessary to remove heat, which is achieved by the evaporation of benzene; For a deeper conversion of ethylene, it is necessary to use increased pressure; The alkylation reaction is a sequential reaction, therefore, to increase the selectivity, it is necessary to maintain the ratio of benzene: ethylene = 3: 1 mol; Aluminum chloride is a weak catalyst, so you should prepare the catalytic complex in advance. Obtaining ethylbenzene is carried out by the method of alkylation of benzene with ethylene. The process of alkylation of benzene with ethylene is catalytic, takes place at a temperature in the range of 125-138 0 C and a pressure of 0.13-0.25 MPa (1.3-2.5 kgf / cm 2), with a thermal effect of 108 kJ / mol. The dosage of raw materials plays an important role in the production of ethylbenzene. Benzene is supplied in an amount corresponding to the established molar ratio of benzene to ethylene of 2.8-3.6: 1. If the ratio of benzene to ethylene is violated, the concentration of ethylbenzene in the reaction mass decreases. High requirements are placed on the drying of the raw material, since moisture leads to deactivation of the catalyst and, consequently, to its consumption. The moisture content in benzene entering the alkylation, it is recommended to maintain at the level of 0.002% (wt.). To do this, the original and return benzene is subjected to drying by azeotropic distillation. The reaction mass (alkylate) formed during the alkylation process contains on average: - 45-60% of the mass of unreacted benzene; - 26-40% of the mass of ethylbenzene; - 4-12% of the mass of PABs (DEB fraction). Corrosion in the production of ethylbenzene is due to the nature of the aluminum chloride catalyst used for alkylation and the process initiator - ethyl chloride. Alkylation products, due to the presence of hydrogen chloride in them, have pronounced corrosive properties, which increase at temperatures above 70 0 C 2.4 Description of the technological scheme of production The process of alkylation of benzene with ethylene is carried out in the alkylator pos. R-1 at a temperature of 125 - 138 0 C and a pressure of 0.13 - 0.25 MPa (1.3 - 2.5 kgf / cm 2). With increasing pressure in the alkylator pos. P-1 is more than 0.3 MPa (3 kgf / cm 2), the supply of benzene and ethylene to the alkylator is stopped. In the alkylator pos. P-1 arrive: Dried benzene charge; Catalyst complex; Faction of DEBs (PABs); Ethylene; Recycled catalyst complex from the sump pos. O-1; Return benzene after the condenser pos. T-1 or pos. T-2; The alkylation reaction proceeds with the release of heat 108 kJ/mol, the excess heat is removed by the circulating catalyst complex and evaporating benzene, which is from the upper part of the alkylator pos. P-1, mixed with off-gases, is sent to the condenser pos. T-1 (pos.T-2) cooled by recycled water. Benzene condensate from the condenser pos. T-1 (pos. T-2) flows by gravity into the alkylator pos. R-1. From the alkylator pos. P-1 the reaction mass enters through the refrigerator pos. T-3, where it is cooled by recycled water to a temperature of 40 - 60 0 C, into the sump pos. O-1 for settling the circulating catalyst complex. The settled circulating catalyst complex from the bottom of the sump pos. O-1 is pumped out to the alkylator pos. R-1. The ratio of the recirculating catalyst complex to the reaction mass in the range of (0.7 - 1.3) : 1 by weight. To maintain the activity of the recycled catalyst complex, the following is provided: Supply of ethyl chloride to the alkylator pos. P-1 and into the line of the recirculating catacomplex. In the event of a decrease in the activity of the recycled catalyst complex, it is provided below for its removal from the sump pos. O-1 for decomposition. From the sump pos. O-1, the reaction mass by self-tex enters the collection of pos. E-1. Alkylate from the container pos. E-1 alkylation unit enters the mixer pos. C-1 for mixing with acidic water circulating in the decomposition system of the catacomplex in the apparatuses: pos. O-2 pos. H-2 pos. С-1 pos. O-2. The ratio of circulating acidic water supplied to the mixer pos. C-1, and alkylate is 2:1. In the decomposition system through the mixer pos. C-1 is also supplied with a used catacomplex (in equal proportions with fresh) after the sump pos. O-1. Settling of alkylate from water occurs in the sump pos. O-2. The excess amount of water from the settling tank, position O-2, according to the level of phase separation, is drained by gravity into the collector of the hydrocarbon stripping unit. The lower water layer from the sump pos. O-2 is recycled to the mixer pos. C-1. Alkylate from the sump pos. O-2 enters the washing column pos. Kn-1 for secondary washing with water supplied from the washing column pos. Kn-2. From the wash column pos. Kn-1 alkylate enters the tank pos. E-3, is pumped out for neutralization into the mixer pos. C-2. The lower aqueous layer from the wash column pos. Kn-3 merges into the container pos. E-2 is fed into the mixer pos. C-1. Neutralization of the alkylate is carried out by a chemical reactant containing NaOH circulating in the neutralization system according to the scheme: pos. O-3 pos. H-5 pos. С-2 pos. O-3. In the sump pos. O-3 sludge occurs alkylate from the reactant solution. The ratio of the circulating solution of alkali and alkylate is 1.2:1. To maintain a constant concentration of the reactant solution in the sump pos. O-3 periodically, according to the results of the analysis, a 15-20% (wt.) solution of the reactant is fed into the line of a circulating 2-10% (wt.) solution of the reactant. Neutralized alkylate from the sump pos. O-3 enters the washing column pos. Kn-2 for cleaning from alkali. Washing of alkylate from alkali is carried out by steam condensate. The bottom layer - chemically contaminated water - from the column pos. Kn-2 enters the collection of poses. E-4, from where it is pumped out for washing the alkylate into the column pos. Kn-1. Alkylate from the wash column pos. Kn-2 flows by gravity into the sump pos. O-4. The bottom layer - chemically contaminated water - from the sump pos. O-4 is drained into an underground tank, and the alkylate enters the tank pos. E-5, from where it is pumped to the warehouse.
Table No. 4.9 Ethylbenzene production waste
Benzene is an organic chemical compound. Belongs to the class of the simplest aromatic hydrocarbons. It is produced from coal tar, during its processing a colorless liquid is obtained, which has a peculiar sweetish smell. Chemical formula - (C6H6,PhH) Benzene is highly soluble in alcohol and chloroform. Perfectly dissolves fats, resins, waxes, sulfur, bitumen, rubber, linoleum. When ignited, it smokes strongly, the flame is bright. Toxic and carcinogenic. It has a narcotic, hepatotoxic and hemotoxic effect. Application at home and at work
It is used for the production of synthetic rubbers, fibers, rubber, plastics. Paints, varnishes, mastics, solvents are made from it. Included in the composition of motor gasoline, is an important raw material for the manufacture of various medicines. Other products are synthesized from benzene: ethylbenzene, diethylbenzene, isopropylbenzene, nitrobenzene and aniline. More recently, benzene was added to motor fuel, but due to the tightening of environmental requirements, this additive was banned. New standards allow its content in motor fuel to be up to one percent, due to its high toxicity. Toxicologists find benzene in foods such as eggs, canned meat, fish, nuts, vegetables, and fruits. Up to 250 mcg of benzene can enter the human body with food per day. How poisoning occurs
Acute poisonings are rare, they can be associated with accidents and accidents at work that have arisen due to violations of safety regulations. So, when cleaning tanks from under benzene, workers can develop lightning death. Once in the body, benzene can cause irritation of the nervous system, profound changes in the bone marrow and blood. A short-term ingress of benzene vapor into the body does not cause changes in the nervous system. If acute poisoning occurs, benzene and its homologues are found in the brain, liver, adrenal glands, and blood. In chronic poisoning, it enters the bone marrow and adipose tissue. It is excreted by the lungs in unchanged form. Symptoms of acute benzene poisoning:
With mild or erased forms of intoxication, changes in the blood picture are hardly noticeable. With severe intoxication, complaints of poor appetite, belching, pain in the right hypochondrium are not uncommon. The mucous membranes and skin become very pale, sometimes spontaneous hemorrhages occur. The liver is greatly enlarged, becomes painful. Decreased acidity and digestion. From the side of the cardiovascular system, myocardial ischemia, tachycardia, and vascular hypotension may begin. The nervous system in severe intoxication reacts differently. Sometimes manifestations of hyperactivity are noted, in other cases lethargy appears, reflexes of the lower extremities decrease Without timely treatment, aleukemic myelosis gradually develops, less often lymphatic leukemia. In the study of bone marrow punctate, the presence of atrophic processes in the bone marrow is detected. In some cases, its complete devastation is observed. In chronic poisoning, which most often develop under industrial conditions, there are changes in the composition of the blood. If the hands often come into contact with benzene, the skin becomes dry, cracks, bubbles, itching, swelling appear on it. First aid and treatmentThe main principle of therapy and prevention of benzene poisoning is the immediate cessation of contact with it at the first symptoms of poisoning. With chronic benzene intoxication, a complete recovery can occur if contact with benzene is stopped in a timely manner. If this is not done, severe intoxication will occur and, despite various methods of therapy, the treatment will be ineffective. When inhaling benzene vapor, doctors note the following clinical picture: there is an excitation similar to alcohol, subsequently the patient loses consciousness, falls into a coma. The face turns pale, convulsions begin, characteristic muscle twitches. The mucous membranes are red, the pupils are dilated. The rhythm of breathing is disturbed, arterial pressure is reduced, the pulse is quickened. There may be bleeding from the nose and gums. In this case, sodium hyposulfite, sulfur and glucose preparations are used, which help to speed up the process of neutralization of benzene and its oxidation products. In case of acute intoxication, it is necessary to ensure the flow of fresh air. The victim is given artificial respiration. When vomiting, glucose is injected intravenously, if blood circulation is disturbed, injections of caffeine are given. Bloodletting, intravenous infusions of glucose, cardiac drugs are carried out. If the patient is too agitated, bromide preparations are used. In severe cases, with pronounced anemia, drugs that stimulate erythropoiesis, vitamin B12, folic acid, iron preparations together with ascorbic or hydrochloric acid are used. Do fractional blood transfusions. Vitamin P is very effective in combination with ascorbic acid. To prevent the development of necrotic phenomena, penicillin and glucose are administered intravenously. In case of toxic hepatitis resulting from chronic benzene poisoning, lipocaine, methionine, and choline are administered. If benzene is taken orally, the clinical picture is as follows: in the mouth and behind the sternum, the patient feels an unbearable burning sensation, severe pain in the abdomen, accompanied by vomiting, excitation, followed by depression. Loss of consciousness may occur, convulsions, muscle twitches begin. Breathing becomes rapid at first, but soon slows down. The smell of bitter almonds is felt from the patient's mouth. The temperature drops sharply. The liver is enlarged, toxic hepatopathy is detected. At very high concentrations of benzene, ingested, the face turns blue, the mucous membranes acquire a cherry red color. A person almost instantly loses consciousness, death occurs within a few minutes. If death does not occur after severe poisoning, health is severely undermined, and often death still occurs after a long illness. If the poison is ingested, the stomach is washed through a probe, vaseline oil, sodium sulfate are injected into the vein, and sodium thiosulfate solution, cordiamine and glucose solution and ascorbic acid are injected into the vein. A caffeine solution is injected subcutaneously. A solution of thiamine, pyridoxine hydrochloride and cyanocobalamin is injected intramuscularly. Antibiotics are prescribed to prevent infection. If there is bleeding, vikasol is injected into the muscle. If the poisoning is mild, rest and warmth are required. PreventionIn production where benzene is used, periodic medical examinations of all workers who come into contact with benzene are required. The therapist, neuropathologist and gynecologist participate in the examination - according to indications. It is not allowed to accept for work in which contact with benzene is possible:
It is forbidden to allow pregnant and lactating women, minors to work with benzene. OBTAINING ETHYLBENZENE Ethylbenzene for the production of styrene is obtained by alkylation of benzene with ethylene according to the reaction: Along with the main reaction, a number of side reactions take place, in which more deeply alkylated benzene derivatives are formed: diethylbenzene C6H6 (C2H5) 2, triethylbenzene C6H6 (C2H5) 3, tetraethylbenzene C6H6 (C2H5) 4. The alkylation reaction is catalyzed by a complex compound obtained on the basis of aluminum chloride, ethyl chloride, benzene and alkylbenzenes: The alkylation reaction proceeds according to the following scheme. Addition of ethylene to the catalytic complex: The exchange reaction between the catalytic complex and benzene with the formation of ethylbenzene: Aluminum chloride can form ternary complexes not only with one, but also with two, three, etc. ethyl radicals, which, when exchanged with benzene, give polyalkylbenzenes. Therefore, in addition to ethylbenzene, the reaction mixture contains diethylbenzene and other polyalkylbenzenes.  The complexes can enter into exchange reactions not only with benzene, but also with reaction products, for example, with diethylbenzene, then the process of transalkylation occurs according to the scheme: Since the transalkylation reaction proceeds simultaneously with alkylation, a fraction of polyalkylbenzenes isolated from the reaction mass during rectification is also fed into the alkylator together with benzene. As a result of all these reactions, a well-defined equilibrium composition of the reaction products is established, depending only on the ratio of alkyl radicals and benzene nuclei in the reaction mixture. Benzene is supplied in an amount corresponding to the molar ratio of benzene:ethylene = (2.8-3.3):1. The reaction mass formed during the alkylation contains on average: 45-55% unreacted benzene, 26-35% ethylbenzene, 4-10% polyalkylbenzenes. The technological process for obtaining ethylbenzene consists of two main stages: alkylation of benzene with ethylene and rectification of the reaction mass. Alkylation of benzene with ethylene The process of benzene alkylation with ethylene is carried out in alkylator 1 (Fig. 37) in an ethyl chloride medium at a temperature of 125–135°C and a pressure of 0.26–0.4 MPa. The following are fed into the alkylator: dried benzene mixture, catalytic complex, polyalkylbenzene fraction, ethylene, recirculating catalytic complex, return benzene.  Rice. 37. 1 - alkylator, 2.3 - condensers, 4 - heat exchanger, 5, 10, 17, 22 - settling tanks; 8, 9, 13, 15, 18, 21, 24 - pumps, 7, 12, 14, 20, 23 - tanks; 8, 16 - mixers, 11, 19 - washing columns. I - benzene, II - ethylene; III - ethyl chloride, IV - catalyst complex; V - polyalkylbenzenes; VI -- spent catalytic complex; VII - stripping and absorption of benzene, VIII - excess water; IX - acid stripping, X - alkaline waste solution; XI - condensate; XII - chemically contaminated water, XIII - reaction mass, XIV - polyalkylbenzenes; XV - neutral blowing. The alkylation reaction proceeds with the release of heat, the excess of which is removed by the evaporating benzene by the recirculating catalytic complex. Benzene from the upper part of the alkylator, mixed with off-gas, is sent to condenser 2, cooled by water. Uncondensed gases from condenser 2 are sent to condenser 3, cooled by chilled water. The blow-offs after the condenser 3 are fed to the further capture of benzene vapors. The benzene condensate from condensers 2 and 3 is drained by gravity down the alkylator 1. From the alkylator 1, the reaction mass through the heat exchanger 4, where it is cooled with water to 40--60 ° C, is sent to the sump 5 to be separated from the circulating catalytic complex. The settled catalytic complex is taken from the bottom of the settler 5 by pump 6 and returned to the alkylator 1. To maintain the activity of the catalyst, ethyl chloride is supplied to the line of the recirculating complex. In the event of a decrease in the activity of the catalyst, the exhausted catalytic complex is decomposed. The reaction mass from the sump 5 is collected in a container 7, from where, due to pressure in the alkylation system, it enters the mixer 8 for mixing with acidic water circulating in the decomposition system: sump 10 - pump 9 - mixer 8. The ratio of the circulating water supplied to the mixer , and the reaction mass is (l-2):1. Water is supplied to the decomposition system from the collector 12 by the pump 13. The reaction mass is settled from water in the sump 10; the lower water layer is sent by pump 9 to the mixer, and the upper layer - the reaction mass - flows by gravity into the washing column and to the secondary washing with water supplied by the pump 21 from the washing column 19. From the washing column 11, the reaction mass flows by gravity into the collector 14, from where pump 15 is pumped out for neutralization into mixer 16. ethylbenzene reaction catalyst production purification The lower aqueous layer from the washing column 11 is drained by gravity into the tank 12 and pumped 13 into the mixer 8. Neutralization of the reaction mass in the mixer 16 is carried out with a 2-10% sodium hydroxide solution. The ratio of the reaction mass and the circulating sodium hydroxide solution is 1:1. The separation of the reaction mass from the alkali solution occurs in the settler 17, from where the reaction mass flows by gravity into the column 19 for washing from alkali with water condensate. The lower layer - chemically contaminated water - is drained from the column into a container 20 and is pumped out by a pump 21 for washing the reaction mass into column 11. The reaction mass from the upper part of the column flows by gravity into the sump 22, then is collected in an intermediate container 23 and pumped to the warehouse. Isolation and purification of ethylbenzene The reaction mass obtained during the alkylation of benzene with ethylene is heated in heat exchanger 1 (Fig. 38) due to the heat of polyalkylbenzenes, in heat exchanger 2 due to the heat of steam condensate, in heat exchanger 3 due to heat exchange with rectified ethylbenzene and in heat exchanger 4 due to the heat of steam condensate and fed into column 5 to separate unreacted benzene. Benzene vapor from the top of the column is condensed in an air condenser 7 and a condenser 8 cooled by chilled water. Uncondensed gases after the condenser 8 are sent to capture benzene. The condensate - return benzene - is collected in a container 9, from where part of it is fed into the column in the form of reflux, the rest is pumped out to the warehouse through the refrigerator 11. The bottom liquid of column 5 is supplied by pump 12 to column 13 to obtain rectified ethylbenzene. The column is heated by steam through an external boiler 14. Vapors of rectified ethylbenzene from the upper part of the column 13 enter the condenser-evaporator 15, where they are condensed due to the evaporation of steam condensate. Uncondensed ethylbenzene vapors are fed into condenser 16. The resulting condensates are collected in tank 17, from where some of them are returned to the column in the form of phlegm by pump 18, and the rest is sent to the warehouse through heat exchanger 3. The bottom liquid of column 13, containing polyalkylbenzenes and resins, is fed by pump 19 to column 20 to separate polyalkylbenzenes from resin. A pair of polyalkylbenzenes from the top of the column 20 is fed to the condensation. The condensate flows into the container 24, from where part of it is fed into the column in the form of reflux, the rest is pumped out to the warehouse through the heat exchanger 1. The polyalkylbenzene resin from the bottom of column 20 is supplied by pump 25 to a warehouse or to a plant for producing copolymers.  Operation mode of the columns of the ethylbenzene separation unit 
Colorless volatile liquid with a specific odor, lighter than water, vapors heavier than air; poorly soluble in water, good in alcohol and other organic compounds. Technical grades of benzene contain xylene and other high-boiling fractions as impurities. Benzene is a dangerous poison. In production, poisoning is possible in emergency situations, in violation of the rules, as well as when working indoors (production of rubber, varnishes, etc.). Poisoning. In high concentrations, benzene causes short-term excitement; then comes general weakness, increased respiration and pulse rate, lowering blood pressure. In low concentrations with prolonged exposure, benzene causes changes in the nervous system, blood, and strikes. The most typical changes in the blood are a decrease in the number of leukocytes, neutropenia, a decrease in the number and a decrease in the content. In the future, aplastic may develop. Sometimes there is an increased sensitivity to benzene in women, especially. Frequent contact with benzene on the skin causes dryness, redness, cracking. Benzene is excreted from the body with exhaled air and urine in unchanged form and in the form of oxidation products. First aid and treatment. In case of acute poisoning with benzene, the victim is taken out to a fresh one. When breathing stops - until spontaneous breathing is restored, oxygen or carbogen, lobelia. contraindicated. With vomiting - a 40% solution intravenously, with circulatory disorders - caffeine. If benzene is ingested, vegetable oil is introduced in order to reduce the absorption of benzene and the stomach is washed (care is required - it is dangerous). With mild poisoning - rest. When excited - drugs. With anemia - transfusion of red blood cells, with leukopenia -,. Administered to prevent infection. Prevention. If the production technology allows, the replacement of benzene with less toxic ones, the sealing of equipment, is optimal. Prevention of pregnant and lactating women, adolescents under 18 years of age from working with benzene. Periodic medical examinations of employees are carried out every six months with a mandatory general blood test. Individual prevention - use (filter or hose with air supply). Ministry of General Education of the Russian Federation KAZAN STATE TECHNOLOGICAL UNIVERSITY NIZHNEKAMSK CHEMICAL AND TECHNOLOGICAL INSTITUTE Department of Chemical technology Group course project Topic: Obtaining ethylbenzene by the method of alkylation of benzene with ethylene Student: Supervisor (_________) Student ka (_________) Nizhnekamsk INTRODUCTION The topic of this course project is the production of ethylbenzene by the method of alkylation of benzene with ethylene. The most common process of petrochemical synthesis is the catalytic alkylation of benzene with olefins, which is determined by the high demand for alkylaromatic hydrocarbons - raw materials in the production of synthetic rubbers, plastics, synthetic fibers, etc. Alkylation is the process of introducing alkyl groups into mo- The reaction of benzene alkylation with alkyl chlorides in the presence of anhydrous aluminum chloride was first carried out in 1877 by S. Friedel and J. Crafts. In 1878, Friedel's student Balson obtained ethylbenzene by alkylation of benzene with ethylene in the presence of ALCL3. Since the discovery of the alkylation reaction, many different methods have been developed to replace the hydrogen atoms of benzene and other aromatic hydrocarbons with alkyl radicals. Various alkylating agents and catalysts have been used for this 48,49. The rate of alkylation of aromatic hydrocarbons is several hundred times higher than that of paraffins; therefore, the alkyl group is almost always directed not to the side chain, but to the core. For the alkylation of aromatic hydrocarbons with olefins, numerous catalysts are used that have the character of strong acids, in particular sulfuric acid (85-95%), phosphoric and pyrophosphoric acids, anhydrous hydrogen fluoride, synthetic and natural aluminosilicates, The relevance of this topic is that in the future styrene is obtained from ethylbenzene by the dehydrogenation of ethylbenzene. 1. THEORETICAL PART 2.1 Theoretical foundations of the adopted method of production. Alkylation of benzene with ethylene. Industrial processes for the alkylation of benzene with ethylene vary depending on the catalyst used. A number of catalysts have been tested on a pilot scale. In 1943, Copers carried out the alkylation of benzene with ethylene on an aluminosilicate catalyst in the liquid phase at 310°C and 63 kgf/cm2 (6.17 MN/m2) at an ethylene:benzene molar ratio of 1:4. The process of alkylation of benzene with ethylene on aluminum chloride at atmospheric or slightly elevated pressure and a temperature of 80-100 ° C has become widespread. Alkylation on a solid phosphoric acid catalyst competes with this method, but only isopropylbenzene can be obtained on this catalyst. Alkylation of benzene with ethylene is practically not carried out on it. A large group of alkylation catalysts are aprotic acids (Lewis acids) - halides of some metals. They usually exhibit catalytic activity in the presence of promoters with which they form products having the character of strong protonic acids. Of the catalysts of this type, aluminum chloride, aluminum bromide, iron trichloride, zinc chloride, titanium trichloride and tetrachloride can be used. Industrial use is only aluminum chloride. On the mechanism of reactions of alkylation of benzene and its homologues with olefins, the following general ideas are adhered to. Alkylation in the presence of aluminum chloride is interpreted according to the mechanism
mu acid catalysis. In this case, the system must have promoter, the role of which is played by hydrogen chloride. The latter may formed in the presence of water: CH3 CH=CH2 + H – CL ∙ ALCL3 ↔ CH3 – CH – CH3 ∙ CL ∙ ALCL3 Further attachment to the aromatic nucleus proceeds according to a mechanism similar to that discussed above: HCL(CH3)2 ∙CL∙ALCL3 +CH3 –CH–CH3 ∙CL∙ALCL3 →HCH(CH3)2 + CH(CH3)2 + CL ∙ALCL3 + HCL + ALCL3 In the presence of aluminum chloride, dealkylation easily proceeds, which indicates the reversibility of the alkylation reaction. Dealkylation reactions are used to convert polyalkylbenzenes to monoalkyl- Thermodynamics of the alkylation reaction. Based on physicochemical constants of hydrocarbons and their thermodynamic functions - enthalpies ΔН and entropy ΔS, you can find the equilibrium constants and calculate the equilibrium yields of alkyl derivatives during the alkylation of benzene with olefins depending on bridges on temperature and pressure. The equilibrium yield of ethylbenzene increases with increasing molar excess benzene and with increasing pressure at a given temperature. C6 H6 + C2 H4 ↔ C6 H5 C2 H5 When benzene is alkylated with ethylene at temperatures below 250-300°C almost complete conversion of benzene to ethylbenzene is achieved. At 450
The alkylation reaction of benzene with ethylene is a first-order sequential reversible reaction. With the deepening of the process, along with monoalkylbenzene, polyalkylbenzenes are also formed. C6 H6 + Cn H2n ↔ C6 H5 Cn H2n+1
Thus, dealkylation is thermodynamically possible with great depth at 50-100°C. Indeed, in the presence of aluminum chloride, it proceeds well, since with this catalyst the alkylation process is reversible. However, at the same temperatures in the presence of acids, dealkylation does not occur at all. M.A. Dalin experimentally studied the composition of the products of benzene alkylation with ethylene in the presence of aluminum chloride. The composition of the reaction mixture is determined by the ratio of benzene and ethylene and does not depend on how the alkylate is obtained: by direct alkylation or dealkylation of polyalkylbenzene. However, this conclusion is valid only when aluminum chloride is used as a catalyst. The alkylation process is carried out in an alkylator - a reaction column lined or lined with graphite tiles to protect against corrosion. Three sections of the column have jackets for cooling, but the main amount of heat is removed by evaporation of some of the benzene. Alkylation is carried out in the presence of a liquid catalyst complex consisting of aluminum chloride (10-12%), benzene (50-60%) and polyalkylbenzenes (25-30%). For the formation of hydrogen chloride, which is the promoter of the reaction, 2% of water from masses of aluminum chloride, as well as dichloroethane or ethyl chloride, the splitting of which produces hydrogen chloride. To isolate ethylbenzene from the alkylate, benzene is distilled off at atmospheric pressure (traces of water are removed simultaneously with benzene). A wide fraction, a mixture of ethylbenzene and polyalkylbenzenes, is distilled off from the bottom liquid at reduced pressure (200 mm Hg, 0.026 MN/m²). In the next column at a residual pressure of 50 mm Hg. (0.0065 MN/m²) polyalkylbenzenes are separated from resins. The broad fraction is dispersed in a vacuum column at a residual pressure of 420-450 mm Hg. (0.054-0.058 MN/m²). Commodity ethylbenzene is distilled in the range of 135.5-136.2°C. To obtain ethylbenzene, ethane is used - the ethylene fraction of pyrolysis containing 60-70% ethylene. Benzene for alkylation should contain no more than 0.003-0.006% water, while commercial benzene contains 0.06-0.08% water. Dehydration of benzene is carried out by azeotropic distillation. The sulfur content in benzene should not exceed 0.1%. The increased sulfur content causes an increase in the consumption of aluminum chloride and degrades the quality of the finished product.
Appendix A shows the process flow diagram for the production of ethylbenzene. The process of alkylation of benzene with ethylene is carried out in the alkylator pos. P-1 in an ethyl chloride medium at a temperature of 125-135C and a pressure of 0.26-0.4 MPa. The following are fed into the alkylator: dried benzene mixture, catalytic complex, polyalkylbenzene fraction, ethylene, recirculating catalytic complex, return benzene. The alkylation reaction proceeds with the release of heat, the excess of which is removed by the recirculating catalytic complex and evaporating benzene. Benzene from the upper part of the alkylator, mixed with off-gas, is sent to the condenser pos. T-1, cooled by water. Uncondensed gases from the condenser pos. T-1 are sent to the condenser pos. Т-2, cooled by chilled water t=0°C. Blowers after the condenser pos. T-2 are sent for further benzene vapor recovery. Benzene condensate from condensers pos. T-1 and T-2 merges by gravity into the bottom of the alkylator pos. R-1. From the alkylator pos. R-1 reaction mass through the heat exchanger pos. T-3, where it is cooled with water to 40-60 ° C, is sent to the sump pos. E-1 to separate from the circulating catalyst complex. The settled catalytic complex from the bottom of the sump pos. E-1 is taken by the pump pos. H-1 and returns to the alkylator pos. R-1. To maintain catalyst activity, ethyl chloride is fed into the recycle complex line. In the event of a decrease in the activity of the catalyst, the output of the spent catalytic complex for decomposition is provided. The reaction mass from the sump pos. E-1 is collected in a container pos. E-2, from where, due to the pressure in the alkylation system, it enters the mixer pos. E-3 for mixing with acidic water circulating in the decomposition system: sump pos. E-4-pump, pos. H-2-mixer, pos. E-3. The ratio of the circulating water supplied to the mixer and the reaction mass is l/2: 1. The lower aqueous layer from the wash column pos. K-1 drains by gravity into the container pos. E-5 and pump pos. H-3 is fed into the mixer pos. E-3. Neutralization of the reaction mass in the mixer pos. E-7 is carried out with a 2-10% sodium hydroxide solution. The ratio of the reaction mass and the circulating sodium hydroxide solution is 1:1. The separation of the reaction mass from the alkali solution occurs in the sump pos. E-8, from where the reaction mass flows by gravity into the column pos. K-2 for cleaning from alkali with water condensate. The bottom layer - chemically contaminated water - is drained from the column into a container pos. E-9 and pump pos. H-4 is pumped out for washing the reaction mass in the column pos. K-1. The reaction mass from the top of the column flows by gravity into the sump pos. E-10, then collected in an intermediate container pos. E-11 and is pumped out by the pump pos. H-7 to the warehouse. Technological scheme for the alkylation of benzene with ethylene on aluminum chloride, which is also suitable for the alkylation of benzene with propylene. The alkylation process is carried out in an alkylator - a reaction column lined with enamelled or lined with graphite tiles to protect against corrosion. Three sections of the column have jackets for cooling, but the main amount of heat is removed by evaporation of some of the benzene. Alkylation is carried out in the presence of a liquid catalyst complex consisting of aluminum chloride (10–12%), benzene (50–60%) and polyalkylbenzenes (25 - 30%). For the formation of hydrogen chloride, which is the promoter of the reaction, 2% of water by weight of aluminum chloride is added to the catalytic complex, as well as dichloroethane or ethyl chloride, upon splitting of which hydrogen chloride is formed.
Alkylation is carried out in a column-type reactor without mechanical agitation at a pressure close to atmospheric (Appendix B). The reactor consists of four tsargs, enameled or lined with ceramic or graphite tiles. For better contact, there is a nozzle inside the reactor. The height of the reactor is 12 m, the diameter is 1.4 m. Each drawer is equipped with a jacket for heat removal during the normal operation of the reactor (it is also used for heating when starting the reactor). The reactor is filled to the top with a mixture of benzene and catalyst. Dried benzene, catalytic complex and gaseous ethylene are continuously fed into the lower part of the reactor. Liquid products of the alkylation reaction are continuously withdrawn at a height of about 8 m from the base of the reactor, and a vapor-gas mixture consisting of unreacted gases and benzene vapor is discharged from the top of the reactor. The temperature in the lower part of the reactor is 100°C, in the upper part it is 90 - 95°C. The catalyst complex is prepared in an apparatus from which the catalyst suspension is continuously fed into the alkylation reactor. Alkilator for the production of ethylbenzene in the liquid phase is a steel column lined inside with an acid-resistant lining pos. 4 or covered with acid-resistant enamel to protect the walls from the corrosive action of hydrochloric acid. The device has four tsargi pos. 1, connected by flanges pos. 2. Three kings are equipped with shirts pos. 3 for cooling with water (for heat removal during the alkylation reaction). The reactor during operation is filled with a reaction liquid whose column height is 10 m . Two coils are sometimes placed above the liquid level, in which water circulates, for additional cooling. The operation of the alkylator is continuous: benzene, ethylene and a catalytic complex are constantly fed into its lower part; the mixture of reactants and catalyst rises to the upper part of the apparatus and from there flows into the sump. The vapors leaving the top of the alkylator (consisting mainly of benzene) condense and return to the alkylator again as a liquid. In one pass, ethylene reacts almost completely, and benzene only 50-55%; therefore, the yield of ethylbenzene per pass is about 50% of theoretical; the rest of the ethylene is lost to the formation of di- and polyethylbenzene. The pressure in the alkylator during operation is 0.5 at(excess), temperature 95-100°C. Alkylation of benzene with ethylene can also be carried out in the gas phase, over a solid catalyst, but this method is still little used in industry. The yield of ethylbenzene is 90 - 95% in terms of benzene and 93% in terms of ethylene. Consumption per 1 ton of ethylbenzene is: ethylene 0.297 tons, benzene 0.770 tons, aluminum chloride 12 - 15 kg.
The cheapest ethylbenzene is obtained by separating it from the xylene fraction of reforming or pyrolysis products, where it is contained in an amount of 10-15%. But the main method for obtaining ethylbenzene remains the method of catalytic alkylation of benzene. Despite the presence of large-scale production of alkylbenzenes, there are a number of unresolved problems that reduce the efficiency and technical and economic performance of alkylation processes. The following disadvantages can be noted: Lack of stable, highly active catalysts for the alkylation of benzene with olefins; Catalysts that have found widespread use - aluminum chloride, sulfuric acid, etc. cause equipment corrosion and are not regenerated; The occurrence of secondary reactions that reduce the selectivity of the production of alkylbenzenes, which requires additional costs for the purification of the resulting products; Formation of a large amount of wastewater and industrial wastes with existing technological schemes of alkylation; Insufficient unit production capacity. Thus, due to the high value of ethylbenzene, at present the demand for it is very high, while its cost is relatively low. The raw material base for the production of ethylbenzene is also wide: benzene and ethylene are obtained in large quantities during the cracking and pyrolysis of petroleum fractions. 3. STANDARDIZATION The following GOSTs were applied in the course project: GOST 2.105 - 95 General requirements for text documents. GOST 7.32 - 81 General requirements and rules for the design of term papers and theses. GOST 2.109 - 73 Basic drawing requirements. GOST 2.104 - 68 Main inscriptions on the drawings. GOST 2.108 - 68 Specifications. GOST 2.701 - 84 Schemes, types, types, general requirements. GOST 2.702 - 75 Rules for the implementation of schemes of various types. GOST 2.721 - 74 Conditional and graphic designations in diagrams. GOST 21.108 - 78 Conditional and graphic representation in the drawings. GOST 7.1 - 84 Rules for the design of the list of references.
4. LIST OF USED LITERATURE. 1. Traven V.F. Organic chemistry: in 2 volumes: textbook for universities / V.F. Traven. - M.: NCC Akademkniga, 2005. - 727 p.: ill. – Bibliography: p. 704 - 708. 2. Epstein D.A. General chemical technology: textbook for vocational schools / D.A. Epstein. - M.: Chemistry, - 1979. - 312 p.: ill. 3. Litvin O.B. Fundamentals of rubber synthesis technology. / ABOUT. Litvin. - M.: Chemistry, 1972. - 528 p.: ill. 4. Akhmetov N.S. General and inorganic chemistry: textbook for universities - 4th ed., corrected. / N.S. Akhmetov. - M .: Higher school, ed. center Academy, 2001. - 743 p.: ill. 5. Yukelson I.I. Technology of basic organic synthesis. / I.I. Yukelson. - M .: Chemistry, -1968. - 820 p.: ill. 6. Paushkin Ya.M., Adelson S.V., Vishnyakova T.P. Technology of petrochemical synthesis: part 1: Hydrocarbon feedstock and products of its oxidation. / Ya.M. Paushkin, S.V. Adelson, T.P. Vishnyakova. - M .: Chemistry, -1973. - 448 p.: ill. 7. Lebedev N.N. Chemistry and technology of basic organic and petrochemical synthesis: textbook for universities - 4th ed., revised. and additional / N.N. Lebedev. - M .: Chemistry, -1988. - 592 p.: ill. 8. Plate N.A., Slivinsky E.V. Fundamentals of chemistry and technology of monomers: textbook. / N.A. Plate, E.V. Slivinsky. – M.: MAIK Nauka / Interperiodika, -2002. - 696 p.: ill.
Introduction……………………………………………………………………………3 2.Technological part………………………………………………………. 2.1. Theoretical foundations of the accepted method of production………….5 2.2. Characteristics of raw materials and the resulting product…………………..9 2.3. Description of the technological scheme………………………………………………12 2.4. Material calculation of production……………………………….15 2.5. Description of the device and the principle of operation of the main apparatus ... .20 3. Conclusions on the project…………………………………………………………….22 4. Standardization………………………………………………………..........24 5. List of literature used……………………………………………25 6. Specification…………………………………………………………………26 7. Appendix A………………………………………………………………………………………………………………………………………………………………………27 8. Annex B…………………………………………………………………28
|
| Read: |
|---|
New
- Meat pigtails - a delicious and beautiful dish of pork Meat pigtails of pork
- Lemon with honey on an empty stomach - action Is a drink from honey and lemon useful?
- Raisins: dried berries, healthier than the grapes themselves Dried raisins
- Bee subpestilence: what is it and what medicinal properties does it have?
- Black sesame: useful properties, contraindications, benefits and harms The benefits of black sesame for a woman's body
- birch leaf tincture
- Products that increase hemoglobin
- Types of fabrics, their properties
- Alkylation at a carbon atom
- Development of the embryo by week: stages, stages









 Benzene is used in the chemical, rubber, printing and pharmaceutical industries.
Benzene is used in the chemical, rubber, printing and pharmaceutical industries. Benzene poisoning occurs through the respiratory system, less often by ingestion and contact with intact skin. The toxicity of benzene is very high, with prolonged interaction chronic intoxication may develop.
Benzene poisoning occurs through the respiratory system, less often by ingestion and contact with intact skin. The toxicity of benzene is very high, with prolonged interaction chronic intoxication may develop. molecules of organic and some inorganic substances. These reactions are of great practical importance for the synthesis of alkylaromatic compounds, isoalkanes, amines, mercaptans and sulfides, etc.
molecules of organic and some inorganic substances. These reactions are of great practical importance for the synthesis of alkylaromatic compounds, isoalkanes, amines, mercaptans and sulfides, etc. ion exchangers, heteropolyacids. Acids in liquid form exhibit catalytic activity in alkylation reactions at low temperatures (5-100°C); acids on solid carriers, for example phosphoric acid on diatomaceous earth, act at 200-300°C; aluminosilicates are active at 300-400 and 500°C and a pressure of 20-40 kgf/cm² (1.96-3.92 MN/m²).
ion exchangers, heteropolyacids. Acids in liquid form exhibit catalytic activity in alkylation reactions at low temperatures (5-100°C); acids on solid carriers, for example phosphoric acid on diatomaceous earth, act at 200-300°C; aluminosilicates are active at 300-400 and 500°C and a pressure of 20-40 kgf/cm² (1.96-3.92 MN/m²).
 -500°C to increase the depth of transformation requires an increase in pressure to 10-20 kgf/cm2 (0.98-1.96 MN/m2).
-500°C to increase the depth of transformation requires an increase in pressure to 10-20 kgf/cm2 (0.98-1.96 MN/m2). C6 H5 Cn H2n+1 + Cn H2n ↔ C6 H4 (Cn H2n+1)2 which are unwanted by-products. Therefore, the composition of the reaction mixture of alkylates is more often determined by kinetic factors than by thermodynamic equilibrium.
C6 H5 Cn H2n+1 + Cn H2n ↔ C6 H4 (Cn H2n+1)2 which are unwanted by-products. Therefore, the composition of the reaction mixture of alkylates is more often determined by kinetic factors than by thermodynamic equilibrium.
 1.2. Characteristics of raw materials and the resulting product.
1.2. Characteristics of raw materials and the resulting product.


 2.3. Description of the technological scheme.
2.3. Description of the technological scheme. Yes, the decomposition system is supplied from the collection of pos. E-5 pump pos. H-3. The reaction mass is settled from water in the sump pos. E-4; lower water layer pump pos. H-2 is sent to the mixer; and the top layer - the reaction mass - flows by gravity into the washing column pos. K-1 for secondary flushing with water supplied by the pump pos. H-4 from the washing column pos. K-2. From the wash column pos. K-1 reaction mass by gravity enters the collection pos. E-6, from where the pump pos. H-5 is pumped out for neutralization into the mixer pos. E-7.
Yes, the decomposition system is supplied from the collection of pos. E-5 pump pos. H-3. The reaction mass is settled from water in the sump pos. E-4; lower water layer pump pos. H-2 is sent to the mixer; and the top layer - the reaction mass - flows by gravity into the washing column pos. K-1 for secondary flushing with water supplied by the pump pos. H-4 from the washing column pos. K-2. From the wash column pos. K-1 reaction mass by gravity enters the collection pos. E-6, from where the pump pos. H-5 is pumped out for neutralization into the mixer pos. E-7.
 1.5. Description of devices and principle of operation of the main apparatus.
1.5. Description of devices and principle of operation of the main apparatus.
 2. CONCLUSIONS ON THE PROJECT.
2. CONCLUSIONS ON THE PROJECT.